Organofunctional Silanes: If you are not using, you are far behind in the league
Organofunctional silanes are hybrid compounds that combine the functionality of a reactive organic group and the inorganic functionality of an alkoxysilane in a single molecule. Most often use organofunctional silanes to bridge the interface between dissimilar materials. These silanes couple dissimilar material through a pair of distinct modes of functionality. They are essential components in adhesive and sealant formulations based on SMP i.e. silane-modified polymers.
Organofunctional silanes are extremely versatile reactive intermediates, widely employed in coatings, adhesives, sealants & elastomers (CASE), inks, and related applications. Selecting the optimal organofunctional silane for an application is dependant on the resin or polymeric material involved, the inorganic filler or substrate and the processing conditions of the application.
Here are some common applications for organofunctional silanes:
- > Adhesion Promoters
- > Improved filler and pigment dispersants
- > Coupling Agents
- > Crosslinking Agents
- > Surface Modification – impart hydrophobic or hydrophilic character
- > Moisture scavenging
Let's see some important and useful applications in polymer systems
Epoxies: Formulators can blend organofunctional silanes into an epoxy resin or employ them by pretreating a filler or surface. Aminofunctional and glycidoxy functional silanes are the preferred organofunctional silanes in epoxy systems such as bisphenol A and bisphenol F diglycidyl ethers. Silane coupling agents improve adhesive properties and mechanical strength and improve dispersity of fillers.
Rubber and Elastomers: Sulfo silane coupling agents in silica reinforced SBR, BR, and natural rubber tire compounds to bridge the dissimilarity of the silica with the hydrocarbon rubber. Silyl ester portion of the coupling agent bond to the silica surface while the sulfo portion of the silane is grafted through the unsaturation in the hydrocarbon rubber. Silica-reinforced green tire compounds exhibit low hysteresis and reduce rolling resistance, thereby contributing to fuel economy.
Polyurethanes: End-modification of polyurethane via silane functionality occurs using either isocyanatosilanes or amino-functional silanes. Isocyanatosilanes are expensive agents, so amino-silanes offer the most cost effective approach to silane modification. Optionally, formulators can pre-treat filler or surfaces with an amino-functional silane prior to compounding with a resin or coating a surface. The improvements in the interfacial strength enable better properties like adhesion, abrasion resistance, durability and mechanical strength. Catalysts like dibutyltin dilaurate increase reaction rates with silyl ester-terminated polyurethanes that moisture-cure in the presence of water or moisture to afford a crosslinked system. Crosslinking improves mechanical properties, durability, weatherability, abrasion and cut resistance.
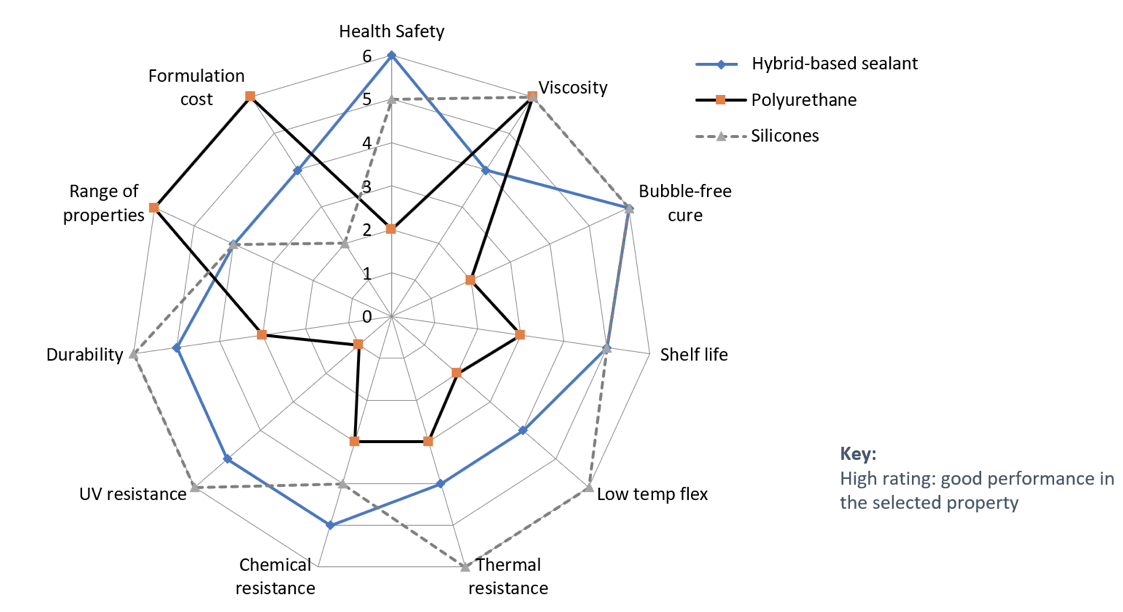
Silyl Ester-Modified Polymers: Silyl-modified polymers (SMPs) are alkoxysilyl functionalized polymers of high importance both academically and industrially. SMPs are being developed for coating, adhesive, sealant and elastomer applications. SMPs are the main components in solvent-free and isocyanate-free sealant and adhesive products. Typically the sealant products manufactured with silyl-modified polymers have good adhesion on a wide range of substrate materials, and have good temperature and UV resistance.
The products cure from a liquid or gel state to a solid. Curing entails crosslinking by the hydrolysis of silyl ethers: 2 RSi(OCH3)2R' + H2O → [RSi(OCH3)R']2O + 2 CH3OH
This hydrolysis generates siloxane linkages. A catalyst is required for this condensation process.
Finally to summarize, we can say that organofunctional silanes fulfill various functions and it's upto you what you want to achieve. You should consider organofunctional silanes because....
-> It permit good adhesion to a variety of different substrates
-> Ensure good storage stability for formulations and prevent premature curing
-> Catalyse the curing reactions of silane-modified polymer systems
-> Improve the physical and mechanical properties of filled and reinforced formulations
-> Enhance the resistance of the cured products to chemicals
Do not forget to check the online training "Using Organofunctional Silanes and Silane Modified Technology For Advanced Formulations In Targeted Applications" to gain formulation expertise in targeted applications.